Behzad Asadollahi*1, Hossein Mahdi2, Ebrahim Goudarzi3, Felora Heshmatpour4
1-Master in Materials Engineering, Head of Quality Lab. of Hamoon Naizeh Co., Asadollahi@hanyco.net
2-Master in Materials Engineering, Head of Quality Lab., Hamoon Naizeh Co., Mahdi@hanyco.net
3-Industry Expert, Quality Assurance Manager, Hamoon Naizeh Company, Goodarzi@hanyco.net
4-Associate Professor, Department of Inorganic Chemistry, Faculty of Chemistry, Khajeh Nasir Toosi University of Technology, heshmatpour@kntu.ac.ir
Abstract
This article investigates the effect material type of pipe on water quality distribution systems in different time frames. A comparative study is conducted between two common types of pipes in water transmission, i.e. ductile iron pipes with cement lining and polyethylene pipes. In this article, the physical and chemical properties of water was studied. Then, the two types of studied pipes, i.e. ductile iron pipes with cement lining and PE, are introduced and their characteristics are reviewed. Sampling at different time points of the water passing through these two different types of water transmission pipes has been done to check the quality parameters. parameters such as; The amount of organic and inorganic chemicals, microbes and bacteria, pH, dissolved oxygen in water and other important parameters are measured. The results of the measurements are analyzed so that the effect of the type and material of the pipe on the quality of water in different time periods is investigated accurately. In this article, the statistical differences and the possible causes of these differences are discussed. This article provides pipeline designers and decision makers in the field of hydrology with important information for choosing the best type of pipe in water transmission systems and can help improve water quality and water resources productivity.
Keywords: Water Quality, Ductile Iron Pipes, Cement Lining, Hygienic Drinking Water, Polyethylene Pipes.
1- Introduction
Today, pipelines and water supply systems are not just used for water distribution; continuous, adequate, and safe drinking water services are important for human health, human rights, well-being, and sustainability (1). In a broader view, ensuring the consumers of health drinking water is crucial in the sense of their citizenship rights. A large portion of the global burden in terms of disease is due to insufficient drinking water services (2).
During the past decades, water pipes have been constructed of steel, cast iron (grey and ductile iron), and even copper, but due to their low cost and availability, they have been replaced by plastics such as polyethylene (PE) and cross-linked polyethylene (PEX). Many newly published reports indicate that plastic pipes can leach significant amounts of various chemicals into water, which can deteriorate the taste and odor of drinking water and lead to many adverse health effects(3). However, information on the possible health effects of using plastic drinking water pipes has been limited (4). The destructive effects of chlorine and other disinfectants on polyethylene pipes have been put into debate by various studies (5).
Many organic additives exist during the manufacturing of plastic pipes (PE, PEX), such as oxygenated compounds: ethyl tert-butyl ether (ETBE), methyl tert-butyl ether (MTBE), and tert-butyl alcohol (TBA). Their degradation product include antioxidant degradation products such as 2, 4-di-tert-butylphenol (2,4 DTBP). It has been shown that solvents benzene, toluene, ethylbenzene, and xylene (4, 6, 7) are readily released from plastic pipes (PE, PEX) upon contact with water. In addition, these molecules are easily washed away, which means that ETBE, MTBE, and TBA can easily change the taste and odor of water (8).
Cast iron pipes have been used in water supply and distribution pipelines around the world since around 1280 AH, and by the advancement of technology, the limitations of gray cast iron (including brittleness, fluid velocity drop, etc.) have been resolved. With the emergence of ductile iron and the application of internal coatings, and the advantages of high durability and green life cycle of ductile iron pipes with internal cement coating, the use of these pipes in water distribution systems with global standards has increased than ever. Ductile iron pipes are presented with various internal coatings of cement types to prevent corrosion of the internal surface of the cast iron. Typically, Pure Aluminum coatings are used in sewage applications. Of course, in some cases, slag cements are used in sewage applications, and resistance to the corrosive environment of sewage may even be provided by increasing the thickness of the internal coating. However, only Portland cement is used to create internal coatings of drinking water transmission pipes. The use of peraluminum cements for drinking water is not recommended due to the amount of aluminum released in drinking water will be very high compared to Portland cements.
Portland cement clinker is obtained by sintering raw materials, including limestone and aluminum silicates, at a kiln temperature of 1450 °C. At this stage of cement production, the elements such as silicon, calcium, aluminum, iron, sulfur, potassium, titanium, magnesium, manganese, and phosphorus enter into the ultimate cement compound. There are usually concerns that heavy metal contaminants, such as lead and mercury etc., enter water from the inner lining of the pipe cement. Meanwhile, in this way, the combustion products of the rotary kiln are combined with the clinker, and heavy metals also enter the cement. These elements in the cement have not been claimed to have a harmful effect on health to date (10). Very small elements such as chromium, lead, zinc, nickel, arsenic, cadmium, vanadium, or copper are released from the cement lining into the water flowing in the pipeline (11).
In studies, there is a possibility of aluminum leaching from Portland cement in alkaline environments (12). The release of heavy metals and harmful elements from cement mortar is a function of the pH of drinking water and the duration of contact of the cement with the internal coating (13).
The subject under investigation is not new, it has been endeavored to avoid laboratory-scale testing and static samples, and to use conditions that are similar to those of real pipelines and to sample circulating fluid. In addition, changes due to water contact with cement were also investigated during months and weeks, concerning the Portland cement coating in one sample, and water contact with plastic pipes in another sample. The present study does not provide comprehensive, general, and fixed information on the effect of pipe type and material on water quality, because the reaction between the coating or inner surface of pipes and water always depends on several variable parameters, the most important of which is the chemical composition of the transported water (14), Therefore, even the most up-to-date laboratory testing methods cannot detect the real complexities of water quality changes in pipelines (under real conditions).
2- Materials and Research Methods
2-1 – Pipes
The present laboratory-based research subjected two 40-centimeter pipes made of ductile iron and polyethylene with the same diameter DN/OD 110 to water circulation so that the desired test water from the outlet of this system could be used as the required sample in quality assessment tests.
Before starting the circulation and water transfer, both 40-centimeter pipe samples (ductile iron and polyethylene) were placed in contact with pure water with the chemical composition as shown in Table 1 for twenty-four hours in accordance with the EN-14120 standard. In addition, 4-millimeter-thick glass was implemented to blind both sides of these pipe samples, which were prepared after cutting a 12-cm diameter of these glasses and heating them to about 450 0C (to eliminate possible contaminants) for two hours.
2-2- Reference Water
The water sample required for the test was prepared by reverse osmosis (RO) purification and removal of possible contaminants, called reference water, for transfer through pipes.
The pure drinking water sample derived after three-stage osmosis purification was considered as a reference sample for examining the chemical composition and was stored in separate glass tanks for each pipe sample of different materials (polyethylene and ductile iron coated with anti-sulfate Portland cement).
2-3- Water Circulation System
The water circulation system used for this study consists of three components, the first is the pipes under study, mentioned in section 2-1. The second is the glass tanks used to store water. The third component or part is the water pump to circulate water from the tank to the pipes and then return the test water from the pipes to the tank, and create this circulation for the desired period. The contact between the tank and the pipes was made using plastic pipes with a 10-mm diameter, which had been washed with pure drinking water for 24 hours before the test. In this system, water was pumped from the tank with a constant flow rate of 1 lit/min into the sample pipe under study by a pump, and then, to complete the water circulation process, it was reintroduced into the primary tank through the path installed at the top of the pipe. The different components of this system are shown in Figure 1.

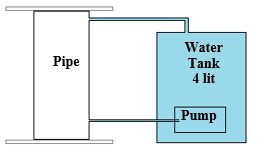

Figure 1- A) Schematic image of the water circulation system – B) Real image of the water circulation system for both pipe samples – C) Image of the pump in the glass tank
2-4- Test Water
After 7 and 30 days of water circulation in these systems, in order to evaluate the quality of this test water in glass bottles that had been previously heated at 450 temperature 0C for 2 hours, it was drained and then transferred to the ViroMed laboratory.
One again, the water was filled in the tank of both sets of 40 cm pipes and the water circulation was started according to a constant flow rate of 1 lit/min by the pump and this situation continued for one month. Then the test water sample was again drained in the glass bottle for testing. All the steps, including heating the glass containers, were also administered for the said test sample.
3- Results and Discussion
3-1- Physical and Chemical Properties
As indicated in Table 1, the physical and chemical properties of the test water sample can be seen for each water circulation system. In addition, these results are related to the results obtained after one week and one month.
Table 1 - Physical and Chemical Properties of Test Results
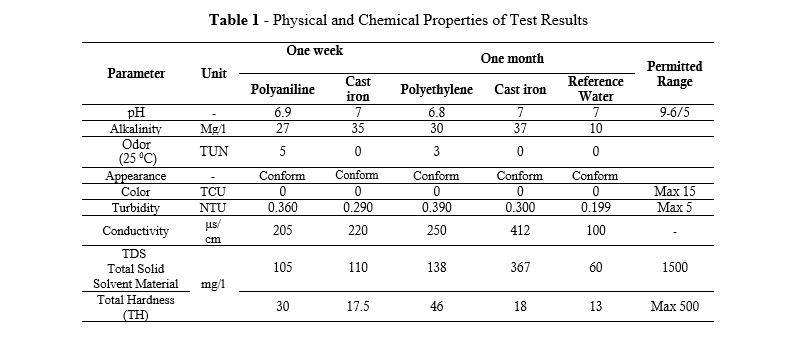
The appearance and color of both test water samples were favorable. For the turbidity parameter of the test water, both samples were in standard permissible conditions, but the turbidity in the test water sample of polyethylene pipes was about 20% higher than the test water of ductile iron pipes having an internal coating of anti-sulfate Portland cement. The total hardness (TH) of the test waters was in the favorable condition, but the case was higher than the total hardness of the test water of ductile iron pipes, for the test water in polyethylene pipes, as shown in Figure 2. The conductivity in the polyethylene test samples was also higher than the ductile iron test water sample, which had an internal coating of cement. The pH of the test water sample in polyethylene pipes increased and then decreased in the first week; the pH changes were noticeable, but in ductile iron pipes having a cement coating, stability in this parameter was seen. The test water sample from polyethylene pipes had an odor exceeding the standard limit. The odor limit at 25°C is 5 TUN units, which reached the maximum allowable range of 7 after one month. A comparison diagram is illustrated in Figure 3.
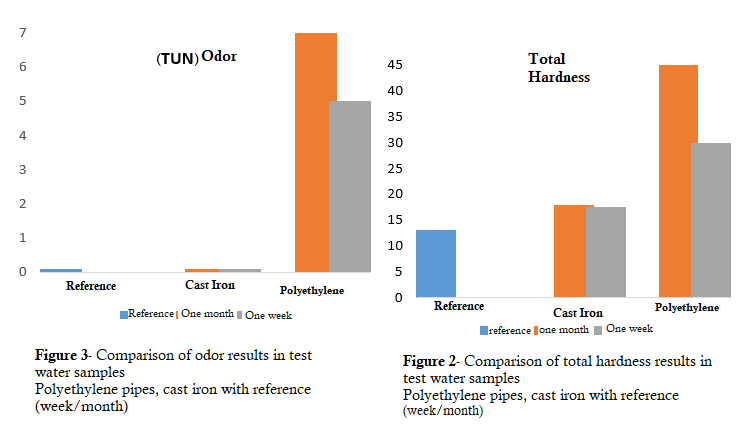
Alkalinity and total dissolved solids in the test water samples of both systems increased compared to the reference water, but were still in a favorable condition compared to the standard permissible range. Alkalinity and dissolved solids in the water samples of ductile iron pipes were higher than those of polyethylene pipes. As Whelton AJ at al (2007) proved in their research (15) that the effect of polyethylene pipes on the chemical quality and odor of water is very serious, this has been confirmed in the results of this study based on Table 1 as well as Figure 2. The water quality status of polyethylene pipes is not in conformity with the water quality standards of Iran and the World Health Organization due to the aforementioned parameters.
3-2- Inorganic Chemicals
The inorganic chemicals measured in the test water samples are listed in Table 2. These results were obtained using the Atomic Fluorescence Spectroscopy Measurement (AFS) method.
Table 2- Toxic Inorganic Chemical Test Results
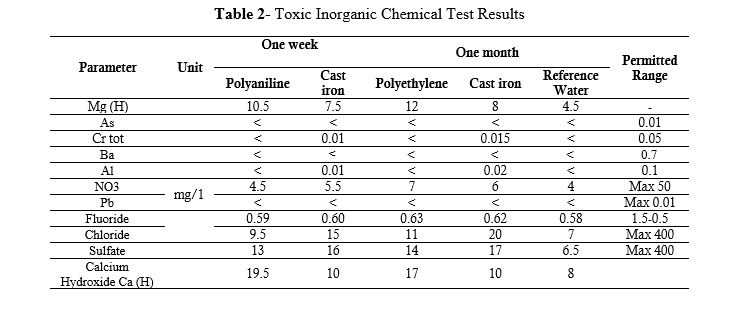
As indicated in Table 2, the elements arsenic, barium, and lead were less than the detection limit of the measurement method, so they are shown with the symbol > in the table of test results. The magnesium element increased in both test water samples compared to the reference water. Although this increase is not significant and the result is within the desired and permissible range, in the test water sample of polyethylene pipes, this increase is greater than the increase in the water sample of ductile iron pipes. As mentioned in the study (X Huang et al., 2020) (16), the deposition of heavy metals in the body of polyethylene pipes is inevitable and causes the destruction of these pipes, and will cause serious health problems by releasing organic carbons. Figure 4 shows the comparative status of magnesium. With nitrate, the test water of both water circulation systems increased compared to the reference water, however this increase is not significant, and the result is within the desired and permissible range. The one-week test water sample of polyethylene pipes has a lower nitrate level than the test water sample of ductile iron pipes, but as a result of testing the test water sample of the same pipes after one month, there was a noticeable increase of 2 units of nitrate, which caused it to be higher than the nitrate in the test water sample of ductile iron pipes after one month. Fluoride, chloride, and alkalinity in the test water sample of both systems increased compared to the reference water, but were still in a favorable condition compared to the standard permissible range. The alkalinity of the water sample of ductile iron pipes was higher than that of polyethylene pipes. Calcium hydroxide in the reference water was 10 mg/L after one week in the test water sample of polyethylene pipes, it increased to 19.5 mg/L, and after one month, it decreased to 17 mg/L. After one week, calcium hydroxide in the test water sample of ductile iron pipes increased by 2 units compared to the reference water, equal to 10 mg/liter. After one month of water cycling in the ductile iron pipe system, this amount remained unchanged and constant. This comparison can be observed in Figure 5.
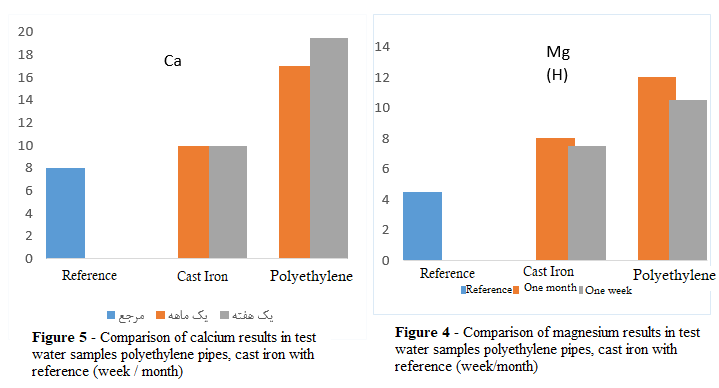
3-3- Organic Chemicals in Water
The results of the test of organic chemicals in the test water samples are listed in Table 3. Dissolved oxygen in water (DO), biological oxygen demand (BOD) (the amount of oxygen required for all organic substances in water to be consumed by microorganisms), and some parameters of chlorinated alkanes and other organic substances such as MTBE, tetrachloroethane, Ethylenediaminetetraacetic acid were also measured.
Table 3 - Results of Organic Chemical Tests in Water
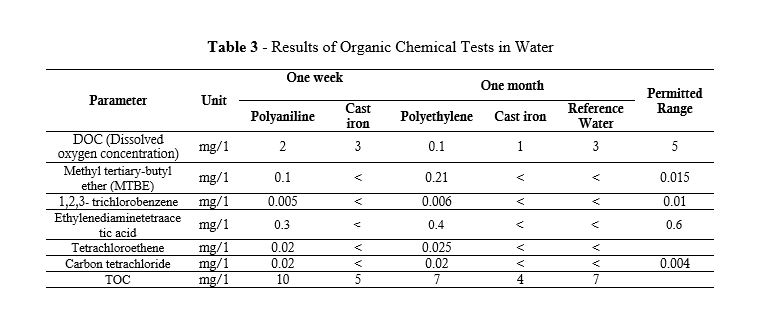
The dissolved oxygen content in the reference water was 3 mg/l, the same content in the test water sample of ductile iron pipes did not change after one week and was the same as that in the reference water. However, this parameter decreased by 1 unit in the test water sample of the polyethylene water circulation system. The decrease in dissolved oxygen content was much more severe after one month.
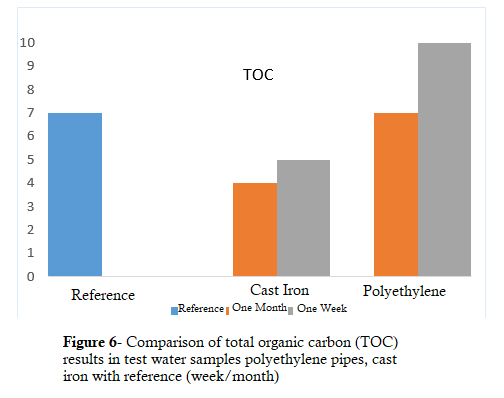
Methyl Tert-Butyl Ether (MTBE), 1,2,3 trichlorobenzene, ethylenediaminetetraacetic acid, tetrachloroethane, and carbon tetrachloride were all trivial in the test water sample of ductile iron and reference water and below the detection limit in the test, but in the test water sample of polyethylene pipes, the value specified in Table 3 was, of course, lower than the permissible limit in drinking water. However, it is related to the amount of MTBE (8), considering the amount of odor present in the sample. The amount of organic carbon (TOC) in water at a temperature of 23 °C increased to 10 mg /L after one week in the polyethylene pipe sample, but decreased to 4 mg / L after one month. In this study, the water temperature was considered to be 25 °C, as Hong et al. (17) in a study they administered in 2020 showed that the release of organic carbon intensifies with increasing temperature, and the released organic carbon reacts with chlorine to produce trihalomethanes (THMS). In the test water sample of ductile iron pipes, however, there was a decrease in the first week, and it decreased to 5 mg /L, and after one month, it decreased to 4 mg/L with a change of 1 unit. The comparison of TOC is illustrated in Figure 6.
3-4- Microbial Characteristics
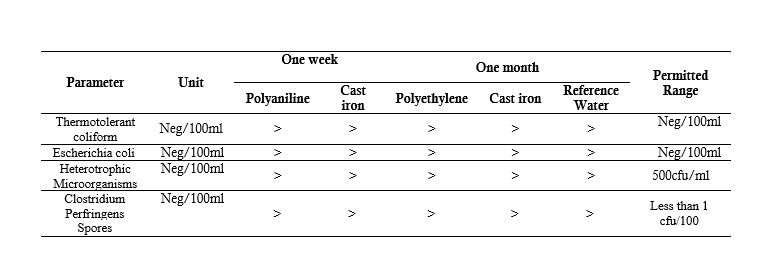
Microorganisms in the test water samples were measured under SMWW standards under numbers 3759, 5271, and 19265. According to the results listed in Table 4, thermophilic coliforms, Escherichia coli, heterotrophic microorganisms, and Clostridium perfringens spores were not observed in a 100-ml sample.
Table 4 - Microbial Test Results
4- Conclusion
As for the physical and visual characteristics of the test water samples, the appearance and color of both test water samples were according to the standards, but the turbidity, total hardness (TH), and conductivity of the test water sample of polyethylene pipes were higher than those of ductile iron pipes with an internal cement coating. The total hardness (TH) of the test water of polyethylene pipes was higher than the total hardness of the test water of ductile iron pipes. The test water sample of polyethylene pipes had an odor exceeding the standard limit. The odor limit at 25 degrees Celsius is 3 TUN units, while in this case, it reached the maximum permissible range of 3 after one month. The reason for this is the release of chemicals from polyethylene pipes into the water, which was not observed in the test water sample of ductile iron pipes. In many studies, the adverse effects of polyethylene pipes on the chemical quality and odor of water have been reported. In some mineral chemical properties, such as sulfate, chloride, fluoride, chromium, aluminum, and nitrate, the test water sample of ductile iron pipes increased very slightly compared to the reference water, which was in a favorable condition compared to the standard. However, this increase was present in the elements magnesium and calcium hydroxide for the test water sample of polyethylene pipes. Although the release of organic carbons in the test water sample of polyethylene was in a small portion, this issue, like many studies that have been conducted in this field, increases the risk of pipe damage and exposure to conditions for the formation of halomethanes, and will ultimately have an adverse impact on the quality of the flowing water.
5- Acknowledgements
Hamoon Naizeh's mission is "water protection," and this article was supported by Hamoon Naizeh Company. The authors herein appreciate Mr. Takian and Mr. Tavakoli (Eng.) for their support. Meanwhile, the author appreciates the fruitful guidance of Ms. Felora Heshmatpour (Eng.D) and Mr. Nemat Hassani (Eng.D).
